
Applied Science Letters


Liver cancer is the fifth most common cancer worldwide in men and women. It is also the second most common cause of cancer mortality rate. Hepatocellular carcinoma (HCC) is one of the most common types of liver cancer. According to the studies worldwide, About 350 million people have chronic HBV disease. At least 60% of HCC cases worldwide are due to chronic hepatitis B virus (HBV) infection. Other non-HBV factors may increase the risk of HCC among persons with chronic HBV infection. Both indirect and direct methods are involved in HCC oncogenesis by HBV. HCC-promoting HBV factors include lasting infection, high levels of HBV replication, HBV genotype, HBV integration, specific HBV mutants, and HBV-encoded oncoproteins (e.g., HBx and truncated preS2/S proteins). Recurrent liver inflammation which is caused by host immune responses during chronic HBV infection can lead to liver fibrosis and cirrhosis, accelerating hepatocyte turnover rate, and accumulating mutations. Major advances in the prevention of HBV-associated HCC with HBV vaccines and antiviral therapies have been made.
Study of the Effect of HBX Gene of Hepatitis B Virus on Liver Cancer Progress
Mahla Khosravi Mashizi 1, Alijan Tabaraei, Golestan2*
1 Master Student in Medical Virology, Golestan University of Medical Sciences, Golestan, Iran
2 Professor, Golestan University of Medical Science, Golestan, Iran.
|
|
ABSTRACT
Liver cancer is the fifth most common cancer worldwide in men and women. It is also the second most common cause of cancer mortality rate. Hepatocellular carcinoma (HCC) is one of the most common types of liver cancer. According to the studies worldwide, About 350 million people have chronic HBV disease. At least 60% of HCC cases worldwide are due to chronic hepatitis B virus (HBV) infection. Other non-HBV factors may increase the risk of HCC among persons with chronic HBV infection. Both indirect and direct methods are involved in HCC oncogenesis by HBV. HCC-promoting HBV factors include lasting infection, high levels of HBV replication, HBV genotype, HBV integration, specific HBV mutants, and HBV-encoded oncoproteins (e.g., HBx and truncated preS2/S proteins). Recurrent liver inflammation which is caused by host immune responses during chronic HBV infection can lead to liver fibrosis and cirrhosis, accelerating hepatocyte turnover rate, and accumulating mutations. Major advances in the prevention of HBV-associated HCC with HBV vaccines and antiviral therapies have been made.
Keywords: Chronic infection, Cirrhosis; Genotype, HBeAg; HBsAg, HBx, Hepatitis B virus, Hepatocellular carcinoma, Integration; Mutation, PreS/S
|
HOW TO CITE THIS ARTICLE: Mahla Khosravi Mashizi, Alijan Tabaraei, Golestan; Study of the Effect of HBX Gene of Hepatitis B Virus on Liver Cancer Progress, Entomol Appl Sci Lett, 2020, 7(3): 55-65. |
Corresponding author: Alijan Tabaraei, Golestan
E-mail * alijant @ yahoo.com
Received: 29/03/2020
Accepted: 17/08/2020
INTRODUCTION
Hepatitis B virus (HBV) is one of the most common viruses that can cause acute and chronic liver disease. Currently, at least two billion people are infected with the virus, and more than 350 million people are infected with chronic infections. One of the serious consequences of chronic HBV is the risk of cirrhosis and hepatocellular carcinoma (HCC) [1]. HBV is a small double-stranded DNA genome virus belonging to the hepadnaviridae family and is the only human hepatotropic virus with the DNA genome. The virus includes four open reading frames (ORFs) including S / preS2 / preS1, C / preC, P and X [2]. ORF is a unique HBx gene that is well conserved in the hepadnaviridae of mammalian viruses. The product of this gene is a protein with 154 amino acids and a molecular weight of 16.7 kDa. It is a multifunctional protein that plays a critical role in the pathogenesis of HBV [3]. HBx can regulate control points in the cell cycle and activate viral cells and genes involved in transcription and regulation of the cell cycle [4]. Regulation of HBx-mediated programmed cell death at control points in the cell cycle has been observed in most cases of hepatitis B virus pathogenesis, such as HCC progression. Evidence indicates a direct relationship between chronic infection and hepatitis B and HCC progress [5]. HBx is produced at low levels in acute and chronic low hepatitis B (CHB). One of the reasons for attributing HBV-induced HCC to HBX protein is that both human hepatitis B virus and marmot have HBX protein and also have a carcinogenic property in their natural host, whereas avian hepatitis B virus lacks HBX protein and has no carcinogenic property [6]. These studies indicate that hepatocellular carcinoma is a viral disease and the studies related to hepatitis B carcinogenic mechanisms have been considered as a key to understanding liver cancer [7]. In a study conducted by Iked et al in 1998, results showed that the HBx gene remains stable in the genome of patients with chronic hepatitis B and this disease may progress towards hepatocellular disease even after treatment [8]. In a study conducted by J Hoare et al in 2001, the results showed that 70% of patients with chronic hepatitis B who underwent liver biopsy contained HBX protein in the cytoplasm of their liver cells. This may indicate the carcinogenic role of HBx [9]. Chung Wook-Tae et al (2003) showed that HBX protein can affect the factors related to survival, proliferation, and growth of cells and cause cancer through its effect on the p53 gene and its inhibition [10]. In a study conducted by Hajiani et al in 2005 in southern Iran, it found that 52.1% of HCC cases were caused by hepatitis B [11].
Molecular Structure of Hepatitis B virus
Hepatitis B virus
Hepatitis B virus (HBV), which is the agent of serum hepatitis B, is a major and the first member of the hepadnaviridae family (DNA-
infected liver viruses). The natural host of HBV is humans [12]. The host range and tissue tendency of these viruses are very limited and the liver is the main site of infection in all of them, but there are few hepadnaviridae DNAs is in the kidney, pancreas, and mononuclear cells. All of these viruses can cause persistent infection.
Although the virus has DNA, it also has the reverse transcriptase enzyme, which is bond to the genome and also has ribonuclease H activity but lacks the integrase activity of retroviruses. Immunization and antigenicity properties of the virus persist after exposure to acid (pH: 2.4: for at least 36 hours), and heat (98 ° C for 1 minute, and 60 ° C for 10 hours), but virus infection is eliminated goes. However, if the virus level is too high, the viruses will not deactivate under the conditions mentioned [13].
Genomic Organization
The hepatitis B virus DNA is a circular double-stranded DNA with a size of 3.2 kb and the virus genome has an unusual structure in which two strands of DNA are not completely symmetric. The negative-strand of DNA is complete and has 3200 bases, encoding the four major viral genes, and it binds to the polymerase by covalent bond at the end of its '5'. The HBV genome has four long open reading frames (ORFs).
Gene X: The smallest gene in the virus, which is probably involved in the transcription rate of other HBV genes and host cell genes [14], directly and indirectly by making HBX protein [14]. HBx can also act as transactivation of many other viral genes and may even be involved in liver cancer [15]. Protein X activity is essential for in vivo replication and viral spread [16]. Protein X reaction with p53 tumor suppressor protein has been suggested as a factor to facilitate the progression of malignant lesions.
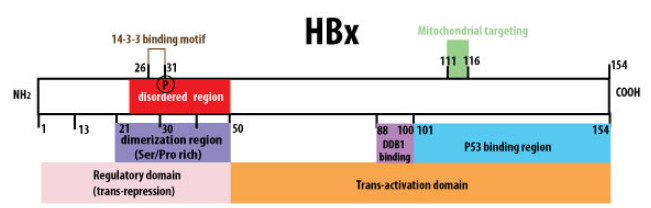
Figure 2: HBx protein stricture scheme (viral zone)
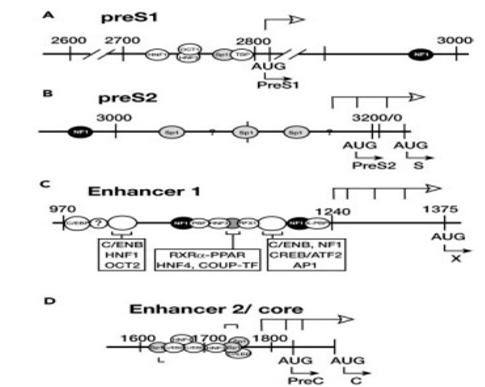
Figure 3: Map of transcription regulation in the hepatitis B virus (Ganem, 2001)
The method of DNA replication of hepadnavirus is crucial and unique because, like retroviruses, reverse transcription of pre-genomic RNA (pgRNA) pattern, and making new DNA strands, is an essential step in the virus replication cycle. To infect the cell, the virus must first attach to the cell surface. It has been proven that the preS1 region of the L-form is involved in HBsAg (HBsAg) and specific virus-cell binding [17]. As the virus enters the cell, the nucleocapsid is transferred to the cytoplasm and then to the nucleus. Within the nucleus of the viral DNA, it is transformed into a type of DNA called supercoiled DNA, which is, in fact, covalent Closed-Circular DNA (CCC), which is a transcriptional pattern for the host RNA polymerase II enzyme, which performs a series of genomic and sub-genomic transcriptions [18]. Viral mRNA is transferred to the cytoplasm to produce a variety of viral proteins such as the core protein, polymerase, and protein X. Then, nucleocapsids are cloned in the cytosol, during which some of the pre-genomic RNA and HBcAg and the DNA polymerase of the hepatitis B virus become immature viral particles. Using the viral DNA polymerase enzyme, a new negative strand of DNA is made from the pgRNA in the capsid. The terminal primase portion of the enzyme starts making a negative-strand and it continues until the end of the strand. As the negative version of DNA is made, all pgRNA, except for a small portion of it, is degraded [19]. The use of the reverse transcriptase enzyme and lack of its correction ability results in high stability of the mutations, while overall genomic compression prevents the high rate of genetic variations [20].
After viral DNA enters the host cell nucleus, the viral DNA is absorbed by several histone proteins and transformed into microchromosomes. It has recently been shown that high levels of replication of the hepatitis B virus are associated with increased acetylation of H3 and H4 histones that are bonded to microchromosomes of the hepatitis B virus. The low levels of the virus inside the blood depend on the low level of acetylation [21].
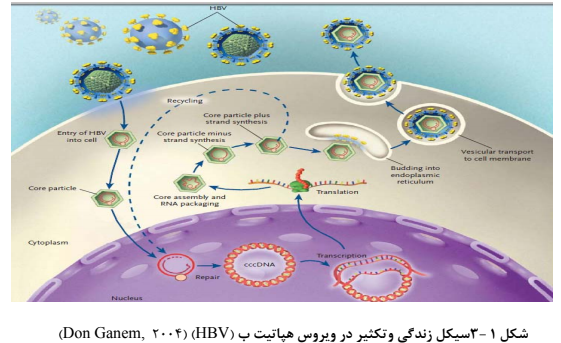
Figure 4: Life cycle and proliferation in hepatitis B virus (Don Ganem, 2004)
The Role of HBx in the Development of Liver Cancer
Protein X is one of the proteins encoded by HBV. It consists of 154 amino acids. Due to its unknown function, it is called protein X. Protein X is required for in vivo infection in the US marmot and increases viral DNA replication in tissue culture in some in vitro conditions [22]. For some reason, it is believed that this protein may be involved in virus carcinogenesis. It can make a complex with the protein P53, which has anti-carcinogenic activity and deactivates it. However, the deactivation process of p53 may occur in a limited number of HBV-induced liver cancers. HBX has been reported to suppress apoptosis induced by beta-TGF p53, TNF, Fas, and genes, leading to death by increased mitochondrial accumulation and release of cytochrome C or sensitization of cells to TNF. It has been also reported that HBX increases cell proliferation and stimulates cell cycle progression by increasing the likelihood of passing the G1-G0 stage. Since HBX can block the differentiated cells in the S Phase, this protein has an important role in controlling the expression of viral and host genes [23]. Another reason to attribute HBV-induced HCC to protein X is that both human and marmot HBV contain protein X and also have a carcinogenic property in their natural hosts, whereas the avian hepatitis B virus lacks protein X and does not have carcinogenicity property in its natural host [24]. HBX expression levels during acute and chronic hepatitis B infection are low and increases cellular and humoral immune responses [25]. HBX has been reported to activate or deactivate regulatory molecules such as microRNAs by post-translational modification (PTS) such as methylation.
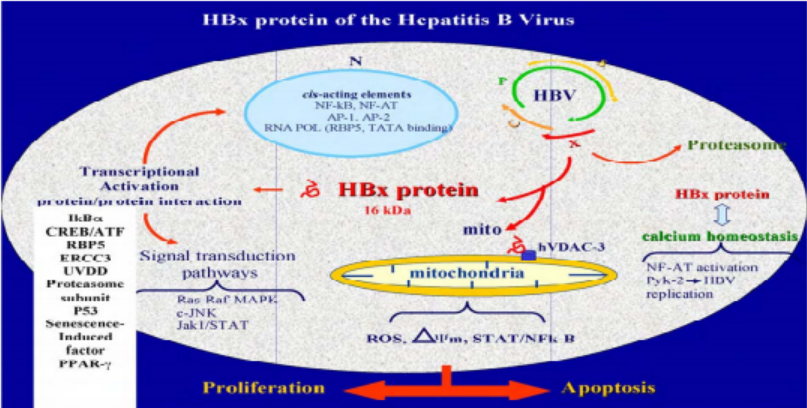
Figure 5: Different functions of Gene X (Kremsdorf, 2006)
Pathogenesis of HBV
Although the initial incidence of hepatitis B virus infection has not been completely clarified, liver injuries likely start by increased cellular apoptosis in cells whose viral antigen is initiated by cytotoxic T lymphocytes (CTLs) infected to the hepatitis B virus.
Clinical studies show that the outcome and severity of the disease depend on host factors and virus variety. The host immune response is the most important factor in the pathogenesis of the disease. One of the most important mechanisms of liver injury is the response of cytotoxic T lymphocytes to infected hepatocytes. Theoretically, it has been widely accepted that cytotoxic T lymphocytes are responsible for the degradation of infected hepatocytes and virus removal [26].
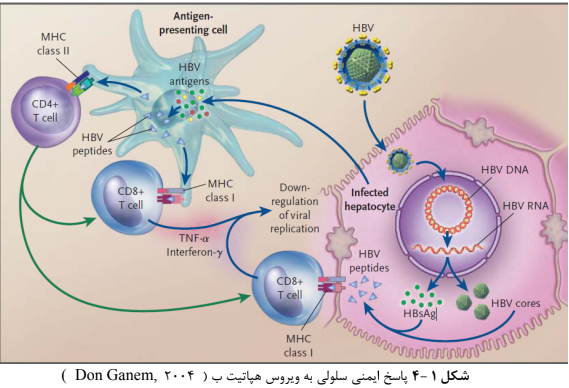
Figure 6: Cellular immune response to hepatitis B virus (Don Ganem, 2004)
Clinical Signs of Hepatitis B
The outcome of hepatitis B infection can vary from severe hepatitis B to very mild or even asymptomatic, depending on the severity of the host immune response to the characteristics of the infectious virus. Acute hepatitis can progress to chronic hepatitis, fulminant hepatitis, cirrhosis, and liver cancer, or lead to a patient’s recovery [27].
Clinical Signs of Chronic Viral Hepatitis
The course of chronic hepatitis B is divided into three stages: 1. Immunological tolerance course: The first stage relates to people who receive the infection from birth and, despite HBV replication, they are asymptomatic and show only a brief illness. At this stage, the liver and spleen are not large and in general, ALT and AST are normal. In liver tissue samples, persistent chronic hepatitis may be seen or maybe completely normal.
Active disease: People who develop HBV during infancy reach this stage at the age of 15 to 35 years. Liver samples show the highest injury, and ALT and AST are high in the blood of patients due to the degradation of hepatocytes. The exacerbated symptoms are seen in the progressive form of the disease and classic clinical manifestations of hepatitis B such as jaundice, anorexia, fatigue, nausea, vomiting, pain in the right and upper abdomen. 2. In the progressive form of the disease, the symptoms are exacerbated and classic clinical manifestations of hepatitis B such as jaundice, anorexia, fatigue, nausea, vomiting, pain in the right and upper abdomen are seen. HBV proliferation rates decrease as the disease progresses. 3. End-stage: In this stage, HBV replication subsides, but the disease is still progressing. In some patients, the histology of the liver remains unchanged, but about 25% of patients with chronic hepatitis are affected with cirrhosis. These patients later progress to liver failure or hepatocellular carcinoma. At the stage where cirrhosis is created, the spleen enlarges, portal venous pressure increases, and the risk of esophageal varices raises. Fluids accumulate in the body and levels of blood factors decrease, and eventually, the patient dies [28].
Cirrhosis of the liver:
One of the possible elements of liver cancer related to the hepatitis B virus is the dynamic course of chronic hepatitis B. liver damage caused by chronic hepatitis B is due to the immune response and the main cause of T cell activity against the virus. However, some data indicate the importance and role of nonspecific chemokines in the secretion of neutrophils and lethal cells, which activate liver injury [29].
High levels of proliferation of liver cells are a risk for the development of liver cancer in cirrhotic individuals. Chronic infection can increase oxidative and secretion of Kupper cells and activates stellate cells. The stable activity of this infection can eventually lead to liver cirrhosis [30]. Cirrhosis is a predisposing condition for liver cancer. In areas where hepatitis B is endemic, patients with liver cirrhosis are approximately three times more likely to develop chronic hepatitis with liver cancer and 16 times more likely to develop symptoms. During cirrhosis, proliferative nodules and fibrosis simultaneously cause severe liver injury and occur in 20% of patients with chronic HBV. Liver cell dysfunction and increased venous blood pressure are some of the most dangerous consequences of chronic HBV infection [31].
Diagnostic tests include:
Measurement of hepatitis B virus antigens and antibodies: These tests can determine the level of immunity against the virus and whether a person has recently been infected with the virus or has a chronic disease in the body and can determine the transmissibility of the disease to others [32].
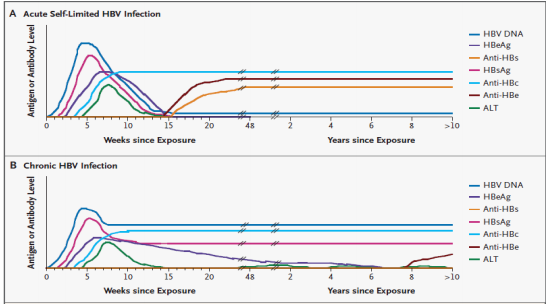
Figure 7: Serological and molecular pattern of markers in hepatitis B infection (Don Ganem 2004)
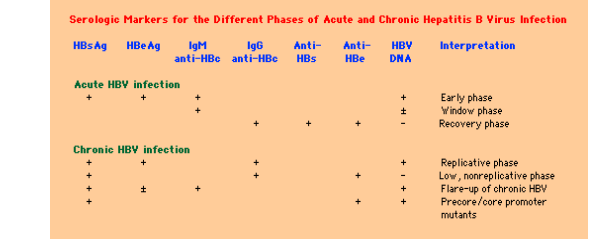
Figure 8: Serological markers for different stages of acute and chronic hepatitis B infection (Don Ganem 2004)
Epidemiology of Hepatitis B
Hepatitis B virus has a worldwide distribution. Although the HBsAg gene is found in primates, humans are still the main host of the virus. In 1998, the number of cases of hepatitis B virus reported by the Centers for Disease Control (CDC) to the National Notifiable Diseases Surveillance System in 1998 was 10258, approximately 3.8 cases per 100000 people [33]. There are more than 350 million chronic HBV carriers worldwide. They make up about 5% of the world's population and 25-30% of them die prematurely due to liver disease. Hepatitis B is prevalent all over the world, but its prevalence is high in China and Southeast Asia and Africa, where carriers are 5% to 20%, while in North America, Western Europe, and Australia, carrier rates are less than 2%. Its average rate in Eastern Europe is 2-7% and in India 4% and Tibet and Nepal, it is very high (61%) and accounting for about 45% of the world population [34].
About 45% of the world’s population lives in areas with a high prevalence of chronic infection, 43% live in regions with moderate prevalence, including Southeast Europe and the Middle East, which the rate of carriers is 2-5%, and 12% were lived in areas with low prevalence. In endemic areas, most patients become infected during childhood, and in highly endemic areas, the rate of chronic infection is 10%. Based on available evidence, 80-90% of the population is transmitted through the perinatal. In regions with moderate prevalence, it is transmitted at all ages, from infancy to adulthood. In countries where the prevalence of HBV is low, viral transmission occurs in adults and in groups who are exposed to infection [35].
DISCUSSION AND CONCLUSION
Two mechanisms show hepatitis B virus is involved in carcinogenesis:
The first mechanism is the entry of the viral genome into the host chromosome causing cis effects that deactivate tumor-suppressor genes or activate tumor-promoting genes. The second mechanism involves the expression of transcription-activated factors that are derived from the hepatitis B virus genome, which has the potential to influence the intracellular transduction signaling pathways and affect the variable expression of housekeeping genes. The entry of hepatitis B virus DNA into host cell DNA during chronic hepatitis virus infection is crucial due to the Promotion or Inhibition of cellular genes that are important for cell growth and differentiation. Also, the expression of hepatitis B virus proteins may have a direct effect on cellular activity and some gene products that can promote cancer. In infected tumor tissues, many hepatitis B virus genes have been found more than others, including pre S / S2 gene, hepatitis B virus X gene, and a new binding transcript called hepatitis B virus binding protein [36]. As a result of the expression of these genes, proteins that are involved in the development of liver cancer are produced. They affect cell growth and death. An important agent in this form of viral activity is protein X, which has multiple activities and is involved in the production of chronically-infected liver cells cancerous. Also, several studies have shown that chronic hepatitis B virus infection is confirmed by the presence of hepatitis B surface antigen in the serum, which is associated with an increased risk of liver cancer [37].
Finally, some liver cancer patients lack detectable serum levels of hepatitis B virus antigen but have low levels of hepatitis B virus DNA in the serum and hepatitis B virus DNA fragments in the cellular genomic DNA (hidden hepatitis B virus infections).
The highest rate of hepatitis B virus infections has been reported in patients with liver cancer, especially among carriers of the hepatitis C virus [38]. It has been suggested that hidden hepatitis B virus infections exacerbate the course of hepatitis C virus infection [39]. Various studies have investigated the prevalence and molecular status of hepatitis B virus infection in liver cancer patients in different regions. In patients with liver cancer and HBsAg negative, hepatitis B virus DNA in the tumor or adjacent non-cancerous tissue was detected by the PCR method in more than half of HBc positive or negative patients [40].
Circular double-stranded DNA from the hepatitis B virus may be detected in the liver of some of these patients, indicating a pattern for transcription and amplification of the viral genome. Some studies have shown that among HBsAg negative patients who have chronic hepatitis B, liver cancer is developed in most areas in hidden hepatitis B virus carriers. One marker of HBsAg liver cancer is X-gene expression in these patients because several studies have shown that positive hepatitis B protein X in liver tissue has reached more than half of liver tissue samples [40]. In all studies, hidden hepatitis B infection and liver cancer, regardless of age and gender, may be associated with hepatitis C virus infection. Viral DNA is associated with the integrated and circular double-stranded genome of the hepatitis B virus in patients with hidden hepatitis virus B infection is associated with genomic amplification and transcription [41]. Also, there is evidence that suggests hidden hepatitis B virus infection is a risk factor for liver cancer and that the potential mechanisms associated with apparent hepatitis B virus infection may cause the growth of tumors that often conceal virus infection [42]. Finally, by designing and making genes against the HBX gene of hepatitis B virus cancer can be prevented.
REFERENCES
