
Applied Science Letters


Background: Some medicinal herbs have excellent antibacterial and cytotoxic effects against bacteria and cancer cells, respectively. Because some of these herbs are potentially toxic to patients, evaluation of their safety before use for treatment is essential. This study aims to evaluate the cytotoxicity effects of Rheum ribes L. (rubarb) extract on cancer cell lines and its antibacterial and mutagenicity activity. Methods: In this study, MTT assay was used to evaluate the cytotoxic effect of Rubarb extract. Also, MBC and MIC were calculated to evaluate the antibacterial effect of this extract. Ames test was used for the potential of genotoxic (mutagenic) assay. Result: The results showed that Rubarb extract had a cytotoxic effect against KB and A549 cancer cell lines at different concentrations (39, 78, 156, 312, 625 and 1250 mg/ml). It also had antibacterial activity against some optional gram-positive and gram-negative bacteria. It had the greatest inhibitory effect against Streptococcus agalactiae. Also, it was shown that this extract did not cause mutation and DNA damage. Conclusion: Finally, using rubarb for the treatment of cancer patients can be a good treatment strategy because of its safety and lack of genotoxicity for normal cells of the body.
The evaluation of cytotoxicity effects of Rheum ribes L. (rubarb) extract on cancer cell lines and its antibacterial and mutagenicity activity
Mozhgan Azadpour1, Mohammad Morad Farajollahi2, Ali Mohamad Varzi3, Frouzan Hadipour3, Mitra Barati1*
1 Research Center of Pediatric Infectious Diseases, Hazrat-e-Rasool Akram Hospital, Iran University of Medical Sciences, Tehran, Iran.
2 Department of Medical Biotechnology, School of Allied Medical Sciences, Iran University of Medical Sciences, Tehran, Iran.
3Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran.
ABSTRACT
Background: Some medicinal herbs have excellent antibacterial and cytotoxic effects against bacteria and cancer cells, respectively. Because some of these herbs are potentially toxic to patients, evaluation of their safety before use for treatment is essential. This study aims to evaluate the cytotoxicity effects of Rheum ribes L. (rubarb) extract on cancer cell lines and its antibacterial and mutagenicity activity. Methods: In this study, MTT assay was used to evaluate the cytotoxic effect of Rubarb extract. Also, MBC and MIC were calculated to evaluate the antibacterial effect of this extract. Ames test was used for the potential of genotoxic (mutagenic) assay. Result: The results showed that Rubarb extract had a cytotoxic effect against KB and A549 cancer cell lines at different concentrations (39, 78, 156, 312, 625 and 1250 mg/ml). It also had antibacterial activity against some optional gram-positive and gram-negative bacteria. It had the greatest inhibitory effect against Streptococcus agalactiae. Also, it was shown that this extract did not cause mutation and DNA damage. Conclusion: Finally, using rubarb for the treatment of cancer patients can be a good treatment strategy because of its safety and lack of genotoxicity for normal cells of the body.
Keywords: Rheum ribes L, Anticancer, Antibacterial, Genotoxicity.
INTRODUCTION
Although the treatment of cancer diseases today has been remarkably effective and has led to the development of effective drugs and therapeutic strategies, the side effects of these drugs are a common problem among patients [1, 2]. The drugs used to treat cancer patients, including chemotherapy, have a series of toxic effects on the healthy cells of patients due to their synthetic and chemical nature [3]. Nature has been the source of therapeutic agents for the remedy of a wide spectrum of ailments all over the world [4, 5]. Medicinal plants are good sources of health-related therapeutic aids to treat diseases [6-8]. Therefore, more recent studies have focused on the use of herbal remedies as a natural extract of herbs for the treatment of cancers [9]. Rheum Ribes L. (Rubarb) is one of the medicinal plants of the family Polygonaceae. Previous studies have shown that this drug has a significant effect on preventing the growth and proliferation of Gram-positive and negative bacteria as well as fungi [10, 11]. However, according to recent studies, Rubarb has been shown to inhibit the growth and proliferation of cancer cells by inducing cytotoxicity and apoptosis and inhibiting cell cycle [12]. Therefore, in this study, we investigated the cytotoxic effect of Rubarb by MTT (methyl tetrazolium) method as well as induction of gene mutation by the AMES TEST on KB and A-549 cancer cell lines.
MATERIALS AND METHODS
Reagent and Cell culture
RPMI 1640 and Fetal Bovine Serum (FBS) were purchased from Biosera (USA). MTT was purchased from Sigma (USA). KB and A-549 cell lines were purchased from the Pasteur institute, Tehran, Iran. Also, Gram-negative bacteria including Pseudomonas aeruginosa (ATCC: 27853), E. coli (PTCC: 1395), Klebsiella pneumonia (PTCC: 1290), and Gram-positive bacteria including Streptococcus agalactiae (ATCC 12386), Staphylococcus epidermidis (ATCC 12228), Staphylococcus aureus (ATCC 25923), and Listeria monocytogenes (ATCC 1298), were purchased from Iranian Pasteur Institute, Tehran, Iran.
Preparation of Plant Extract
Rubarb stalks were collected from the Damavand Mountains around Tehran and dried in the shade after washing. The dried plant was crushed in a mortar and soaked in ethanol (80%) for 72 hours. Then, the mixture was passed through a filter paper and dried at 45 °C.
MTT assay
MTT assay is used to evaluate the cytotoxicity of the extract on KB and A-549 cell lines. 1× 105 KB and A-549 cells were cultured in each well of 96-well plate separately. In order to determine the cytotoxicity effect of the extract, cell lines were treated at different concentrations (39, 78, 156, 312, 625, 1250, 2500, and 5000 μg/mL) for 24 and 48 h under 5% CO2 and 95% humidity situation. After incubation, 20 μL (5μg/ml) of MTT was added to each well and the cells were incubated at 37 ° C for 4 h, then 100 μL DMSO was added to each well and the well was incubated in dark for 15 min, finally, absorbance was measured at 570 nm by ELISA reader to assess the percentage of cell viability.
Antibacterial Susceptibility Assay
The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of each bacterium were determined by Micro dilution broth method [13]. In this way, a serial dilution of the plant extract was prepared in a liquid medium (39- 2500µg/ml). Then, 100 μl of each dilution was added to each well of 96-well micro-plate, respectively. The bacterial suspension was prepared with standard turbidity (equivalent to half McFarland), and 100 µl was added to each well. The plate was incubated at 37 °C for 18 to 24 hours. Finally, 15 μl of the substance 5-3.3-phenyltetrazolium chloride was added to all wells at a concentration of one hundred percent, and then the plates were incubated at 37 °C for 3h. The last well in which no growth was observed to be the lowest concentration of the plant extract was considered as MIC. Also, Vancomycin for gram-positive and gentamicin for gram-negative bacteria was used as positive controls. MIC of positive control for gram-positive and negative bacteria were recorded at 8 and 16 µg/ml respectively in Table 2. All experiments were performed for each bacterium as triplicate.
AMES mutagenicity Assay
The potential genotoxic effects of Rubarb species was investigated using histidine-requiring strains of Salmonella typhimurium TA100 [14]. The procedure was that 100 µl of different concentrations (5000, 2500, and 1250 µg/plate) Rubarb solution was first added to tubes containing 2 ml top agar and 0.1 ml TA100 strain overnight and then 30 min incubation in 37 °C. The contents of the tubes were then uniformly dispersed on the surface of the glucose agar plates after 3 seconds of shaking. After tightening, agar plates were inverted and incubated at 37 °C for 48 hours. DMSO solution was used as negative control and sodium azide was used as positive controls. The extract was tested in the absence (-S9) or presence (+S9) of rat liver microsomal fraction, to which 20 ml of S9 liver homogenate mix per plate was added. After incubation of culture for 48 h at 37 °C, the mutated colonies were counted on a colony counter.
Statistical analysis
In this study, descriptive statistics were used to analyze the data of cytotoxic effect and the ANOVA test was performed for inferential statistics. P-value <0.05 was considered significant.
RESULTS
Cytotoxicity effect
MTT assay was used to evaluate the cytotoxic effect of Rubarb extract. As can be seen in Figure 1, different doses of Rubarb extract reduced growth and viability of KB and A549 cell lines. Data analysis showed that the cytotoxic effects of Rubarb extracts on KB cells increased after 24 h at concentrations above 312 μg/ ml, which was statistically significant (p-value <0.001). In the case of A549 cells, the results showed that the cytotoxic effect of Rubarb extract was at a concentration of 156 µg/ml greater than compared to 39 µg/ml, which was statistically significant (p-value <0.0001). After 48 hours, the cytotoxic effect of the extract was increased on both KB and A549 cells, which was dependent on the concentration of the extract (Figures 1 and 2).
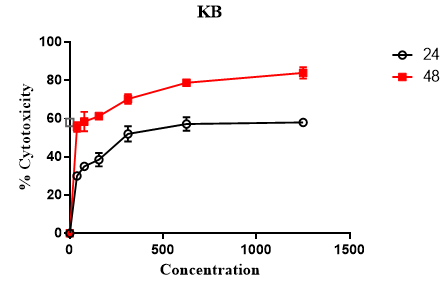
Figure 1. Cytotoxic Effect of Rubarb Extract on KB cell lines
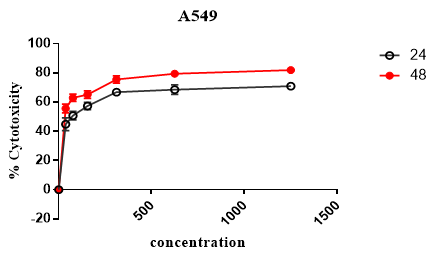
Figure 2. Cytotoxic Effect of Rubarb Extract on A549 cell lines
Evaluation of Antibacterial Properties of Rhubarb Extract
In this study, we investigated the antimicrobial activity of Rubarb stalk extract on optional gram-positive and gram-negative bacteria. The results are presented as MIC and MBC in Table 2. The MBC level for all bacteria except Ps. aeruginosa and S. aureus was 500 µg/ml. The MIC for E. coli, S. aureus, and K. pneumonia 500, for Ps. aeruginosa and S. epidermidis 250, but for S. agalactiae and L. monocytogenes was 125 μg/ml. MIC results showed that the antimicrobial effect of Rubarb stalks on Streptococcus agalactiae and Listeria monocytogenes was higher than other bacteria (Table 1).
Table 1. The Antimicrobial Effects of Hydro-alcoholic Extracts of Rubarb stalk on Optional gram-positive and gram-negative Bacteria.
|
Listeria monocytogenes |
Staphylococcus epidermidis |
Staphylococcus aureus |
Streptococcus agalactiae |
K. pneumonia PTCC: 1290 |
E. coli PTCC: 1395 |
P. aeruginosa ATCC: 27853 |
Drug |
|||||||
|
MBC |
MIC |
MBC |
MIC |
MBC |
MIC |
MBC |
MIC |
MBC |
MIC |
MBC |
MIC |
MBC (μg/ml) |
MIC (μg/ml) |
|
|
500 |
125 |
500 |
250 |
- |
500 |
500 |
125 |
500 |
500 |
500 |
500 |
- |
250 |
Rubarb stalk Extract |
|
8 |
2 |
8 |
4 |
16 |
8 |
4 |
2 |
|
|
|
Vancomycin |
|||
|
|
|
|
|
|
128 |
8 |
64 |
8 |
5 |
5 |
Gentamicin |
|||
Evaluation of Mutagenic Properties of Rhubarb Extract
Ames test was used to evaluate the mutagenicity of Rubarb using TA100 strain. Different concentrations of Rubarb extract (1250, 2500, and 5000 µg/plate) were used in this study. The results showed that the number of TA100 colonies increased with increasing extract concentration. It was also found that the number of colonies at concentrations containing S9 was higher than those without S9 (Table 2).
Table 2. Mutagenicity effects of Rubarb stalk on S. Typhimurium TA100.
|
With S9 |
Without S9 |
Concentration* |
||||
|
1250 |
2500 |
5000 |
1250 |
2500 |
5000 |
|
|
208± 7.63 |
230± 7.34 |
303± 6.18 |
255± 6.16 |
316± 7.76 |
386± 9.79 |
Stem extract |
|
0.44 |
0.48 |
0.63 |
0.97 |
1.2 |
1.48 |
MI |
|
|
|
993± 4.5 |
|
|
821±6.9 |
NaN3 2.5ϻg/ plate** |
|
|
|
476± 9.79 |
|
|
260± 2.89 |
DMSO 100ϻg/ plate*** |
MI= Colony count in presence of extract/Colony count in presence of blank
*µg/ plate**1.5 µg/ plate***100µg/ plate
DISCUSSION
Today, cancer is one of the leading causes of death worldwide. Recently, significant advances in the treatment of patients have been made through the use of appropriate therapeutic strategies [15]. In contrast, some of these strategies, such as chemotherapy with side effects for patients, have been limited in their use for the treatment of patients [16]. Therefore, the use of medicinal herbs is increasing due to their natural ability to treat cancer patients. Recent studies have shown that these herbal remedies have a significant role in inhibiting cancer cell proliferation and growth through cytotoxic induction [17, 18]. Rubarb is one of the herbs that is used for its cytotoxic effect to treat many diseases [19]. A study by Keser et al. showed that the extract of Rubarb stalk had a cytotoxic effect against the cell lines PC-3, A2780, HCT-116, and MCF-7 [20]. A study by pembegul et al. to investigate the cytotoxic effect of Rubarb extract showed that Rubarb increased induction of HL-60 cell line apoptosis by increasing caspase-3 expression and decreasing BCl-2 expression [21]. In the present study, our results showed that the use of Rubarb stalk and leaf extract increased cytotoxicity against KB and A549 cell lines (Table 1). Although the use of herbal medicines has been associated with a reduction in side effects compared to chemotherapy drugs, some of these drugs have been shown to be associated with mutations in patients, which may be the cause of cancer itself. Therefore, in this study, we investigated the mutagenic effect of Rubarb extract [22]. The results showed that the number of colonies in the presence of Rubarb extract containing rat liver microsomes (S9) was higher than that without S9. However, the ratio of the number of colonies grown on a culture medium to blank was less than 2, indicating that the extract was safe and non-mutagenic (Table 3). However, a study by Abudayyak et al. showed that a concentration of 25 mg/ml of Rubarb extract caused a mutation in DNA [23].
Antimicrobial activity was another characteristic of Rubarb extract that was evaluated in this study. After statistical analysis, it was shown that Rubarb extract inhibited the growth of some optional aerobic and anaerobic gram-positive and gram-negative bacteria (Table 2). These results were similar to the study by Amiri et al., which showed that Rubarb extract inhibited the growth of some Gram-positive and negative bacteria [23]. However, a study by Alaadin et al. showed that Rubarb extract at concentrations of 4000 and 250 mg/mL did not inhibit Ps. aeruginosa and E. coli, respectively [24].
CONCLUSION
The side effects of chemotherapy drugs have led to more therapeutic approaches to the use of herbal remedies for the treatment of cancer patients. The results of this study showed that Rubarb had a cytotoxic effect against KB and A549 cancer cell lines. Also, since it has been shown that the use of this herbal medicine has no potential for mutagenic effect, it can be said that the use of Rubarb in the future can be used for the treatment of patients as it is safe and has a cytotoxic effect on cancer cells.
ACKNOWLEDGMENTS
The authors appreciate the support of the Research Center of Pediatric Infectious Diseases, Hazrat-e-Rasoul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran. Financial support for this project was provided by the Iran University of Medical Sciences, Tehran, Iran. Project ID: 95-01-131-28128
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All the procedures performed in the studies involving human participants were in accordance with ethical standards of local ethics committee of Iran University of Medical Sciences as well as 1964 Helsinki declaration. Written informed consent was obtained from all patients and normal subjects.
REFERENCES
