
Applied Science Letters


Drought stress is one of the main influencing factors for rice plants. The impacts of PGPRs were examined in relation to drought resistance. The plant growth-promoting rhizobacteria (PGPR) Bacillus and Pseudomonas sp. were tested for their role in increasing plant growth in cultivars of rice (Oryza sativa) under different level of drought stress. Qualitative analyses of Indole Acetic Acid (IAA) and Gibberellins were carried out at drought stress. After the inoculation of IAA and Gibberellins into the crop field, it improved crop productivity. Plant growth-promoting bacteria Bacillus and Pseudomonas sp. Were immobilized by alginate bead inoculants and later dried and deposited at ambient temperature for 14 years. When inoculated onto rice plants, Bacillus and Pseudomonas spp. can colonize and create plant growth effects and survive in alginate inoculants over long periods. This impact of PGPRs application improves drought tolerance of rice under water deficit conditions.
Drought Tolerant and Comparative Analysis of PGPRs in Susceptible Cultivars of Oryza Sativa Plant
R. Pandeeswari, S. Uma Devi*
Department of Microbiology and Biochemistry, Nadar Saraswathi College of Arts and Science, Theni - 625 531, Tamil Nadu, India.
ABSTRACT
Drought stress is one of the main influencing factors for rice plants. The impacts of PGPRs were examined in relation to drought resistance. The plant growth-promoting rhizobacteria (PGPR) Bacillus and Pseudomonas sp. were tested for their role in increasing plant growth in cultivars of rice (Oryza sativa) under different level of drought stress. Qualitative analyses of Indole Acetic Acid (IAA) and Gibberellins were carried out at drought stress. After the inoculation of IAA and Gibberellins into the crop field, it improved crop productivity. Plant growth-promoting bacteria Bacillus and Pseudomonas sp. Were immobilized by alginate bead inoculants and later dried and deposited at ambient temperature for 14 years. When inoculated onto rice plants, Bacillus and Pseudomonas spp. can colonize and create plant growth effects and survive in alginate inoculants over long periods. This impact of PGPRs application improves drought tolerance of rice under water deficit conditions.
Keywords: PGPRs, Pseudomonas sp., Oryza sativa, rice, water deficiency, lipid
INTRODUCTION
Rice is a major staple food [1-3] devoured by more than one-third of the world’s population [4], giving up to 80% of the day by day calories admissions of an endless larger part of the human population, particularly in Asia [5]. Rice has semi-aquatic nature and is grown under the overflowed condition to supply bulky amounts of water. Half of the rice regions within the world do not keep up overflowed condition, and in this way, the yield is decreased, to a few degree, as a result of drought [6]. Rice with little adaptation to water-restricted condition is strikingly delicate to drought stress [7]. Drought could be major abiotic stress debilitating agricultural generation around the world [8]. To address this worldwide challenge in agriculture, an investigation has focused on the move toward germplasm to extend water utilization productivity [9]. In any case, later consideration has turned to the application of useful microorganisms that intervene drought resistance and move toward plant water use productivity, and these efforts have been increased due to mechanical advances in microbiomics [10]. Microbes can likely survive under drought conditions because of their capacities to deliver phytochemicals. The application of plant growth-promoting rhizobacteria (PGPR) is well-known for their growth-promoting properties like production of phytohormones, capacity to solubilize mineral phosphate and to antagonize plant pathogens. A rhizobacterium qualifies as PGPR when it exhibits development-promoting properties like the generation of phytohormones Indole Acidic Acid (IAA) and Gibberellic acid [11]. The amount of IAA may be an appropriate marker for bacterial viability especially under osmotic stress [12]. PGPR like Bacillus and Pseudomonas recently have received attention as inoculants to resist plants under changed biotic and abiotic stress conditions because of their fabulous root colonizing capacity [13]. Bacillus spp. have been demonstrated to be perfect candidates for the improvement of productive natural items due to their capacity to produce heat resistant endospores. The utilization of microorganisms for plant growth promotion and disease control is well recognized [14].
Inoculation of plants with microorganisms to improve yields of crops or local plants has been practiced for a few decades. It is well-known that inoculants’ properties are essential for effective inoculation with plant growth-promoting microbes. Alginate beads have been detailed to protect the useful properties of PGPRs [15]. The capacity of dry alginate beads for long-term survival, viability, and growth-promoting of Bacillus and Pseudomonas were assessed for 14 years. In the present study, we chose the Bacillus and Pseudomonas strains because of their long-term survival and growth-promoting capacity under drought-stress environment and used Alginate beads as a carrier by supplementation. The results were compared with the results of free cell inoculums in promoting the development of Rice plants.
MATERIALS AND METHODS
Soil sample
The soil was collected from the Paddy field in Ammapuram, Vadapudupatti, Theni area, Tamilnaidu. The soil was taken in sterile conditions in polythene packs at 4°C. It was air-dried for isolation of bacteria.
Isolation and biochemical characterization
Isolation of bacteria was done by multilevel dilution procedures. Distinctive biochemical test viz, catalase test, starch hydrolysis, triple sugar press agar (TSI), indole test, methyl ruddy test (MR test) and Voges-Proskauer test (VP Test) were conducted to identify the chosen isolates as depicted in Bergey’s Manual of Determinative Bacteriology.
Phytohormone Analysis
Determination of IAA by Spectrophotometer
IAA generated by the bacteria was determined by a colorimetric strategy with the addition of Salkowski reagent. Bacterial isolates were inoculated into ten ml nutrient broth (NB) medium containing 1% L-tryptophan and NB media without L-tryptophan. The cultures were incubated and shaken (80 rpm) at room temperature for two days. Three ml of each culture was transferred into two microtubes and centrifuged (10,000 rpm) for 15 minutes. A total of two ml of the filtrate obtained was transferred into a sterile test tube and two ml of Salkowski reagent was added. At that point, the suspension was kept for one hour in dark at room temperature. IAA levels were determined utilizing a spectrophotometer at a wavelength of 530 nm [16].
Determination of Gibberellins by Spectrophotometer
Gibberellins were determined utilizing the standard strategy of Borrow. Isolates were grown in NB, incubated at room temperature for one week, centrifuged for ten minutes at a speed of 8000 rpm. The culture was transferred into a fifteen ml tube and two ml of zinc acetate solution was added. Then, two ml of potassium ferrocyanide solution was added and centrifuged for ten min at 8000 rpm. Five ml of supernatant was added to five ml of 30% hydrochloric acid and kept at 27°C for 75 minutes. The absorbance was estimated utilizing a spectrophotometer at 430 nm. The concentration of Gibberellin was determined after comparing it with the standard curve of Gibberellins [17].
Alginate Beads:
The cell immobilization technique described by Bettmann and Rehm [18] was utilized. Ten milliliters of overnight (O/N) cultures were admixed with 90 ml sterile 3% sodium alginate. The mixture was added by a five ml sterile syringe into a sterile 2% calcium chloride solution. The beads were allowed to harden for thirty min, rinsed with distilled water and collected.
Pot Experiment
Paddy seeds were mixed with rice boiled water and allowed to dry for a few minutes. This served as a binder for the bacterial cells. Then, the seeds were mixed with Bacillus and Pseudomonas spp. cultures and allowed to stand for one hour. The seeds were sown in pots containing sterilized soil.
Plant Growth Parameters
From each pot, a random seedling was taken and the necessary measurements were carried out. The crops were harvested carefully after maturity, rinsed with tap water, and length of roots and shoot (cm) were determined.
Analysis of Plant Phytochemical
Protein content
Total protein content was determined by the method of Lowry et al. [19]. For all the treated and untreated plants, 0.1 g of the leaves were added to two ml of distilled water and crushed well using a mortar and pestle. The extract was centrifuged at 3000 rpm for ten minutes. One ml of the supernatant was taken and utilized in the measurements.
Carbohydrate content
For determining total carbohydrate, 0.1g of leaf sample was added to two ml distilled water and smashed well using a mortar and pestle. The extract was centrifuged at 3000 rpm for ten minutes. One ml of the supernatant was taken and utilized in the measurements.
Chlorophyll content
Total chlorophyll content was determined by the method of Arnon [20]. Leaf samples were homogenized in 80% acetone using a mortar. The extract was centrifuged at 5000 × g for 5 min. The absorbance of the supernatant was recorded at 663 and 645 nm by spectrophotometer (Nicolet Advancement 100, Thermo Logical, USA). The chlorophyll content was calculated using the formula: Chlorophyll Substance = 20.2 × OD value at 663 nm) + 8.02 × OD value at 645 nm.
RESULTS AND DISCUSSION
Biochemical characterization
Bacteria were isolated from paddy soil. Table 1 shows the results of biochemical characteristics and cell morphology of the isolated bacteria. The bacterial isolates were identified as Bacillus and Pseudomonas.
Phytohormones analysis:
Indole Acetic Acid (IAA) Production
Quantitative analysis was carried out by using a spectrophotometer at 530 nm, and a great quantity of production of IAA was observed.
Table 1: Biochemical Characterization
|
Biochemical Characterization |
Sample 1 |
Sample 2 |
|
Gram Staining |
+ |
- |
|
MR test |
- |
- |
|
VP test |
+ |
- |
|
Indole test |
+ |
+ |
|
Citrate |
+ |
+ |
|
Gelatine |
+ |
+ |
|
Starch |
+ |
+ |
|
Catalase |
+ |
+ |
|
Oxidase |
- |
+ |
|
TSI test |
+ |
+ |
|
Cetrimide |
- |
+ |
|
Skim milk |
+ |
- |
MR: Methyl red test, VP: Voges–Proskauer test, TSIA: Triple Sugar Iron Agar test.
The phytohormones analyses results were given in Table 2 and the highest gibberelilin and IAA production was observed in Pseudomonas and Bacillus respectively. The highest value of IAA production was obtained by Bacillus (46 µl per 50 ml of medium) followed by Pseudomonas (41 µl per 50 ml of medium).
Gibberellins Production:
A quantitative analysis was carried out by using a spectrophotometer at 254 nm, and a great amount of gibberellin production was observed. Table 2 shows that results originated from qualitative and quantitative tests of gibberellins. The table reflects the capacity of two microorganisms which revealed a color with a small variation in intensity [21]. Within the quantitative estimations, the highest value of IAA production was obtained by Pseudomonas (85 µl per 50 ml of medium) followed by Bacillus (70 µl per 50 ml of medium).
Table 2: Phytohormones analysis
|
Sample |
IAA production (530 nm) |
Gibberellins production (254 nm) |
|
Bacillus |
46µg/ml |
70µg/ml |
|
Pseudomonas |
41µg/ml |
85µg/ml |
Pot experiment
The study phytohormones namely IAA and gibberellins, growth parameters including root and shoot length, plant phytochemicals including protein, carbohydrate, and chlorophyll were noted and presented in Fig. 1. In all the study parameters concerning plant growth, there was a significant increase in all treatments compared with the control.
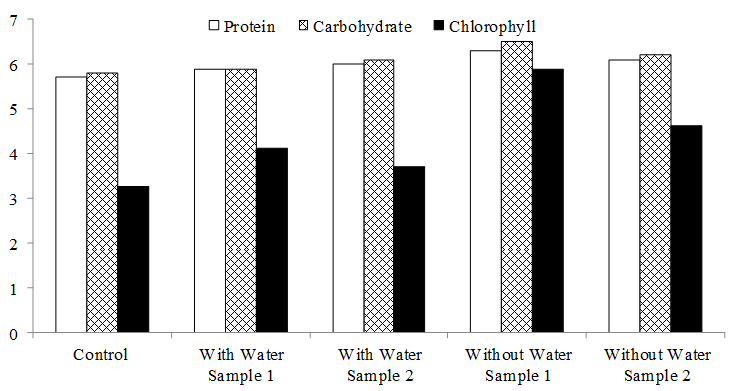
Fig. 1: Comparative analysis of Phytochemicals in paddy plants inoculated with Bacillus and Pseudomonas.
Plant Growth Parameters:
With respect to plant root and shoot length, the control plant produced minimum root and shoot length (2.8 cm and 4.8 cm, respectively) compared with treated plants after 30 days of transplantation. In treated plants, without water sample 2 produced maximum root and shoot length (3.8 cm and 6.3 cm respectively). In study results, the highest root and shoot length was recorded for without-water sample (4.3 cm and 8.2 respectively) (Fig. 2).
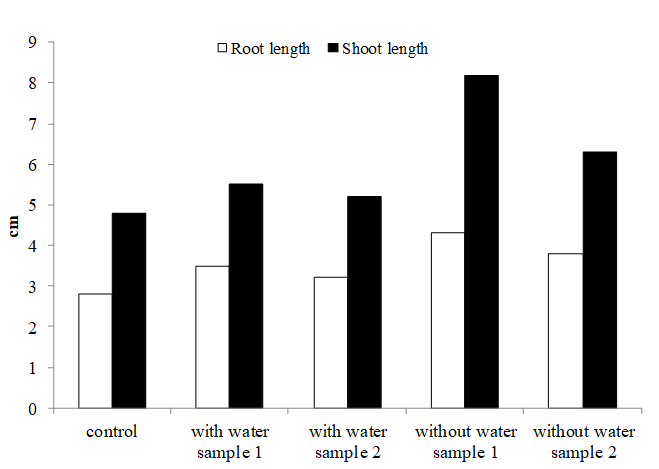
Fig. 2. Comparative analysis root and shoot length paddy field isolates Bacillus and Pseudomonas
Phytochemical analyses
Protein concentrations for treated and untreated leaf extracts were noted by using a spectrophotometer at 620 nm. After 30 days of seed inoculation, the protein content was estimated. The control paddy leaf produced minimum protein (1g of leaf produced 5.7 mg) as compared to treated plants. In this study, the highest protein content was noted in without water sample 1 (1g of leaves produced 6.3mg). After 30 days of seed inoculation, the carbohydrate content was estimated. The control paddy leaf produced minimum carbohydrate (1 g of leaf produced 5.8 mg) as compared to treated plants. In this study, the highest carbohydrate content was noted in without water sample 1 (1 g of leaves produced 6.5 mg) [8]. After 30 days of seed inoculation, the chlorophyll content was estimated. The control paddy leaf produced minimum carbohydrate (1g of leaf produced 3.26g) as compared to treated plants. In this study, the highest carbohydrate content was noted in without water sample 1 (1g of leaves produced 5.88g) (Table 3).
Table 3: Phytochemical analysis
|
S. NO. |
Sample |
Weight of leaf (g) |
Quantity of Protein (g) |
Quantity of Carbohydrate (g) |
Quantity of chlorophyll (g) |
|
1. |
Control |
1 |
5.7 |
4.8 |
3.26 |
|
2. |
With Water Sample 1 |
1 |
5.9 |
5.8 |
4.11 |
|
3. |
With Water Sample 2 |
1 |
6.0 |
5.2 |
3.71 |
|
4. |
Without Water Sample 1 |
1 |
6.3 |
6.5 |
5.88 |
|
5. |
Without Water Sample 2 |
1 |
6.1 |
6.0 |
4.43 |
CONCLUSION
Drought stress is one of the greatest issues confronting the world nowadays. It leads to decrease in crop yields and loss in the amount of arable lands. The percentage of drought stress were increasing due to climate change and their rate of increamnet was different in the location. Different approaches have been built up to reduce drought impacts on crop plants. But due to the drawbacks related to these procedures like high cost and potential negative natural side effects, the success of these procedures is questionable. Using low cost, environment-friendly Bacillus and Pseudomonas inoculation can be a valuable approach to overcome the negative impacts of drought and lead to improved crop yield under drought stress. Mechanism of drought tolerance in rhizobacteria and qualities capable of these mechanisms can advance research prospects. Plant-microbe interaction to moderate drought tolerance at the genetic, atomic, and biochemical levels are dynamic issues to be addressed in future studies.
ACKNOWLEDGMENT
The authors are thankful for providing research facilities by the Department of Microbiology and Biochemistry, Nadar Saraswathi College of Arts and Science, Theni.
REFERENCES
