
Applied Science Letters


Introduction: High-cholesterol diet is one of the major causes of cardiovascular disease leading to the death of millions of people annually. One of the ways to prevent this risk is the use of chemical drugs, but herbal compounds at controlled levels have less adverse effects than chemical compounds. In the present study, we investigated the effect of hydroalcoholic extract of a saffron petal on malondialdehyde (MDA) and superoxide dismutase (SOD) enzymes, inflammatory markers and lipid profile and compared it with lovastatin in hypercholesterolemic rats. Materials and Methods: Thirty adult Wistar rats were randomly divided into 6 equal groups (n = 5). Group 1 (sham) received a normal diet and Group 2 (control) received only a high-cholesterol diet (2%). Group 3 to 6 were treated with a high-cholesterol diet (2%) in the first 4 weeks and 100, 200, and 300 mg/kg of saffron petal extract and 10 mg/kg of lovastatin, respectively, in the second 4 weeks. At the end of the study, the level of activity of antioxidant enzymes and the level of lipid profile and inflammatory markers were measured. Results: The mean plasma MDA level, SOD enzyme activity of red blood cell, lipid profile and inflammatory markers were significantly increased in the control group (high-cholesterol diet) compared to the sham group (P <0.001). However, these cases in the other groups treated with hydroalcoholic extract of the saffron petal (groups 3, 4 and 5) and lovastatin drug (group 6) showed a significant decrease (minimum significant difference, P <0.05), despite receiving high-cholesterol diet (p<0.05) compared to the control group. Conclusion: The results of this study showed that a non-toxic dose of saffron petal extract has an effective role in preventing cardiovascular diseases by reducing the risk factors associated with these diseases.
Investigating the Effect of Saffron Petal Extract on Antioxidant Activity and Inflammatory Markers in Hypercholesterolemic Rats
Mojtaba Mohamadpour 1, Parastoo Shahmir2, Masoumeh Asadi3, Sirous Asadi1, Mansour Amraei4*
1Student Research Committee, Faculty of Medicine, Ilam University of Medical Sciences, Ilam, Iran.
2Department of Clinical Biochemistry, Faculty of Medicine, Ilam University of Medical Sciences, Ilam, Iran.
3Department of Physiology, Faculty of Medicine, Ilam University of Medical Sciences, Ilam, Iran.
4Biotechnology and Medicinal Plants Research Center, Ilam University of Medical Sciences, Ilam, Iran.
ABSTRACT
Introduction: High-cholesterol diet is one of the major causes of cardiovascular disease leading to the death of millions of people annually. One of the ways to prevent this risk is the use of chemical drugs, but herbal compounds at controlled levels have less adverse effects than chemical compounds. In the present study, we investigated the effect of hydroalcoholic extract of a saffron petal on malondialdehyde (MDA) and superoxide dismutase (SOD) enzymes, inflammatory markers and lipid profile and compared it with lovastatin in hypercholesterolemic rats. Materials and Methods: Thirty adult Wistar rats were randomly divided into 6 equal groups (n = 5). Group 1 (sham) received a normal diet and Group 2 (control) received only a high-cholesterol diet (2%). Group 3 to 6 were treated with a high-cholesterol diet (2%) in the first 4 weeks and 100, 200, and 300 mg/kg of saffron petal extract and 10 mg/kg of lovastatin, respectively, in the second 4 weeks. At the end of the study, the level of activity of antioxidant enzymes and the level of lipid profile and inflammatory markers were measured. Results: The mean plasma MDA level, SOD enzyme activity of red blood cell, lipid profile and inflammatory markers were significantly increased in the control group (high-cholesterol diet) compared to the sham group (P <0.001). However, these cases in the other groups treated with hydroalcoholic extract of the saffron petal (groups 3, 4 and 5) and lovastatin drug (group 6) showed a significant decrease (minimum significant difference, P <0.05), despite receiving high-cholesterol diet (p<0.05) compared to the control group. Conclusion: The results of this study showed that a non-toxic dose of saffron petal extract has an effective role in preventing cardiovascular diseases by reducing the risk factors associated with these diseases.
Keywords: saffron, Malondialdehyde, Superoxide dismutase, Inflammatory markers, Rat.
INTRODUCTION
Diseases such as heart attack, angina pains and myocardial infarction result in the hospitalization of millions of people annually, and unfortunately, many of them die [1]. Inflammation plays a major role in all stages of atherosclerosis from onset to progression and causes events such as plaque rupture and, consequently, acute vascular dysfunction [2]. Some of the inflammatory markers include C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), interleukin β1 (IL-β1), and IL-6, which their levels change in the progression of cardiovascular diseases [3, 4]. Inflammation is caused by increased oxidative pressures, which usually results in elevated CRP levels [5]. Also, elevated cholesterol levels can increase the levels of this nonspecific inflammatory protein [6]. Oxygen radicals play a major role in the development of atherosclerosis. Antioxidants and hypolipidemic compounds inhibit atherosclerosis [7, 8].
Drugs are known as "statins" are prescribed to lower and treat blood lipids. Statins are one of the most commonly prescribed drugs in the world [9]. Statins generally inhibit competitively HMG-CoA reductase (β-Hydroxy β-methylglutaryl-CoA reductase) and prevent the conversion of HMG-CoA reductase to mevalonate. As a result, the production of mevalonate as a precursor to cholesterol synthesis in hepatocytes is inhibited. In a reactive reaction, LDL receptors that are present on the surface of liver cells increase significantly and accordingly the serum LDL-C level decreases. In addition to lowering cholesterol, statins lower serum lipid levels [10]. However, these drugs have many medicinal complications, such as myopathy, rhabdomyolysis, decreased liver enzymes, nausea, dizziness, and digestive problems, increased risk of cancer, liver damage and increased risk of type II diabetes [11-15]. Statins also inhibit the coenzyme Q10 biosynthesis that is needed for energy production [16, 17]. For this reason, the use of statins has clinical limitations.
It has been also shown that the use of medicinal herbs has a great impact on reducing blood lipids [18]. This positive effect is due to the presence of polyphenol compounds and their flavonoids, which may be due to their antioxidant effects [19, 20]. The antioxidant activity of flavonoids causes these compounds to counteract the destructive effects of reactive oxygen species such as superoxide radicals and to protect the cells [20]. Saffron is one of the foods that contain flavonoids [21]. Glycine flavonol myricetin, kaempferol, quercetin and two types of anthocyanins called delphinidin and pentamidine are compounds found in saffron petals [22]. The most abundant antioxidant among the active compounds of saffron is a water-soluble carotenoid called crocin [23, 24]. Various studies have shown that this herb has various medicinal activities such as antioxidant, antidepressant, blood pressure-lowering, blood glucose-lowering, anti-cancer, anti-ischemia, anti-hyperlipidemia, sex enhancer, gene protecting, memory enhancer, and anti-poisoning activities and it inhibits morphine withdrawal syndrome and diazinon antibody [25-30].
Although saffron is considered as one of the most expensive food spices in the world, its petals are not used extensively and are discarded. Due to a limited number of studies on saffron petals compared to its stigma and also the frequency and low cost of saffron petals compared to stigma and as most of the herbal compounds have less toxic and adverse side effects at controlled levels compared to chemical compounds, the present study investigated the effect of hydroalcoholic extract of saffron petal on SOD and MDA enzymes (antioxidant activity indices), inflammatory markers and lipid profile and compared these effects with the effects of lovastatin drug in hypercholesterolemic rats.
MATERIALS AND METHODS
Ethical Considerations
The present study was approved with a certification letter for medical research that was issued by the ethics committee of the Ilam University of Medical Sciences with the code number of (IR.MEDILAM.REC.1397.042).
Extraction
Saffron petals were collected from the saffron fields around Mashhad in the autumn, which is the harvest season of this plant, and were identified and approved. Then, they were dried in a cool place without light. A certain amount of the powder was mixed with water and ethanol alcohol (1: 4 ratio) and placed in an incubator shaker (34 °C and 140 rpm) for 72 hours. The refined mixture was transferred to a rotary machine by Watman paper for condensation. The distilled extract was poured into clean Petri dishes to obtain a dry extract in the oven at 30-40 °C. Finally, the dried extract was stored at the appropriate refrigerator temperature for use in the next experiments.
Toxicity Determination
The toxicity level of the hydroalcoholic extract of the saffron petal was determined by assessing the lethal dose of this extract. For this purpose, 50 to 3200 mg/kg/day (progressive increase) of this extract was administrated as gavage to seven groups of animals and 2 ccs of normal saline were administrated to the eighth group. After 24 hours, the rate of mortality was measured in all groups, and finally, LD50 and LD100 levels of extract were calculated using computer techniques [31-34]. LD50 level for a hydroalcoholic extract of the saffron petal was determined at 670 mg/kg [35] and 100, 200, and 300 mg/kg/day doses of hydroalcoholic chamomile extract (15, 30 and 45% LD50) were used in this study.
Grouping and treatment
Thirty male Wistar rats purchased from Pasteur Institute of Tehran were used in this study (200 ± 30 gr). These laboratory animals were kept in specially maintained cages at a temperature of 25 ± 2 °C and a 12-h light cycle. They had free and unrestricted access to water and food. The animals were kept at these conditions for one week to adapt to the laboratory environment. Then, they were divided into 6 groups: Group 1 (Sham) received a daily diet and 2 ccs of normal saline (n = 5). Group 2 (control) received high-cholesterol diet (2%) (n = 5). The two groups also received 2 ccs of normal saline daily due to being kept at similar conditions. Groups 3, 4 and 5 received a high-cholesterol diet (2%) and were treated with 100, 200, and 300 mg/kg/day doses of hydroalcoholic extract of saffron petal, respectively (n = 5). Group 6 received high-cholesterol diet (2%) and were treated with 10 mg / kg / day of lovastatin (n = 5). The treatment lasted for 8 weeks.
Biochemical factors and their measurement:
At the beginning and the end of the study, blood samples were taken from all animals in the different groups, while they were fasting for 12 hours. Then, the taken blood was placed at laboratory temperature and clotted after half an hour. Then, it was centrifuged at 4500 rpm for 5 min to prepare serum. Finally, biochemical kits were used to assess plasma malondialdehyde (MDA) concentration, erythrocyte superoxide dismutase (SOD), inflammatory markers (IL-6, CRP, and TNF- α) and lipid profile (HDL, LDL, TG, and cholesterol).
Statistical analysis
SPSS version 16 software and one-way analysis of variance (ANOVA) and Tukey's test were used to compare the means of a variable in different groups. The mean level of variables for each group was calculated as Means ± SD and P<0.05 was considered as a significant level.
RESULTS
Determining the dose of hydroalcoholic extract of saffron petal
The results of determining the toxicity showed that the LD50 level of hydroalcoholic extract of the saffron petal was about 670 mg/kg. Accordingly, we used 15%, 30% and 45% of the LD50 dose of extract, that is, doses of 100, 200, and 300 mg/kg/day.
Lipid profile
In this study, a high-cholesterol diet (2%) at the end of the study significantly increased serum levels of cholesterol, TG and LDL-c in Group 2 (control) compared to Group 1 (sham) (P<0.001). As shown in Figure 1, in Groups 3 to 6 (despite receiving high cholesterol food), treatment with different doses of hydroalcoholic extract of saffron petal (Groups 3, 4 and 5) and lovastatin (Group 6) caused a significant decrease in serum levels of cholesterol, TG and LDL-c compared to Group 2 (only high-cholesterol diet) (minimum significant difference, P<0.05). At the end of the study, serum level of HDL-c in Group 2 (control) that received high-cholesterol diet (2%) was significantly lower than that of Group 1 (sham) (P<0.001), but a significant increase was found in serum level of HDL-c in Groups 3 to 6 compared to Group 2 (control) (minimum significant difference, P<0.05) (Figure 1).

Figure 1. Comparison of serum levels of lipid profile in different groups.
#: Comparing the control group with the sham group, *: Comparing other groups with the control group. The mean difference is significant at the 0.05 level (#,*: P<0.05).
Inflammatory Markers
The results of this study showed that a high-cholesterol diet (2%) at the end of the study significantly increased the serum levels of inflammatory factors (IL-6, CRP, and TNF- α) in Group 2 (control) compared to Group 1 (Sham) (P<0.001). Although Groups 3 to 6 received high-cholesterol diet, treatment with different doses of hydroalcoholic extract of saffron petal (Groups 3, 4, and 5) and lovastatin (Group 6) caused a significant decrease in serum levels of these inflammatory factors in these groups compared to Group 2 (high-cholesterol diet only) (minimum significant difference, P<0.05) (Table 1).
Table 1. Comparison of serum levels of inflammatory factors in different groups.
|
Inflammatory Indicators |
Group 1 (Sham) |
Group 2 (Control) |
Group 3 (Extract 100 mg/kg/day) |
Group 4 (Extract 200 mg/kg/day) |
Group 5 (Extract 300 mg/kg/day) |
Group 6 (Lovastatin 10 mg/kg/day) |
|
CRP (ng/mL) |
1.83 ± 0.08 *** |
3.59 ± 0.19 ### |
2.61 ± 0.06 ** |
2.07 ± 0.10 *** |
1.99 ± 0.10 *** |
2.13 ± 0.11 *** |
|
TNF-α (pg/mL) |
11.31 ± 0.21 *** |
18.41 ± 0.30 ### |
15.83 ± 0.16 * |
12.52 ± 0.21 *** |
12.21 ± 0.17 *** |
11.91 ± 0.17 *** |
|
IL6 (ng/L) |
2.88 ± 0.05 *** |
4.78 ± 0.09 ### |
4.08 ± 0.14 * |
3.39 ± 0.06 ** |
3.11 ± 0.09 *** |
3.41 ± 0.09 ** |
|
Fibrinogen (mg/mL) |
2.45 ± 0.07 *** |
4.06 ± 0.09 ### |
3.49 ± 0.09 * |
3.33 ± 0.08 * |
2.61 ± 0.10 *** |
3.01 ± 0.12 ** |
#: Comparing the control group with the sham group, *: Comparing other groups with the control group. The mean difference is significant at the 0.05 level (#,*: P<0.05).
Antioxidant activity
The results also showed that the level of SOD of red blood cells in the Group 2 (control) with high cholesterol-diet had a significant increase (P<0.001) at the end of the study compared to Group 1 (Sham). However, in the other groups receiving the hydroalcoholic extract of the saffron petal (Groups 3, 4 and 5) and lovastatin arbitration (Group 6), it showed a significant reduction compared to the control group (minimum significant difference, P<0.05 (Figure 2).
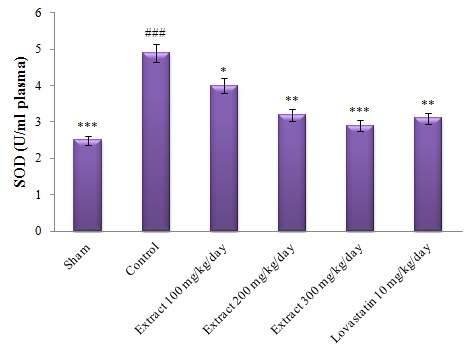
Figure 2- Comparison of plasma MDA levels in different groups.
#: Comparing the control group with the sham group, *: Comparing other groups with the control group. The mean difference is significant at the 0.05 level (#,*: P<0.05).
As shown in Figure 3, this significant difference is also observed in plasma levels of MDA, so that high cholesterol-diet in the control group significantly increased plasma levels of MDA compared to the sham group at the end of the study (P<0.001). However, treatment of other groups with hydroalcoholic extract of saffron petal (Groups 3, 4 and 5) and lovastatin drug (Group 6) resulted in a significant reduction (minimum significant difference, P<0.05), in plasma levels of MDA in these groups compared to control group, despite receiving high-cholesterol diet by these groups (Figure 3).
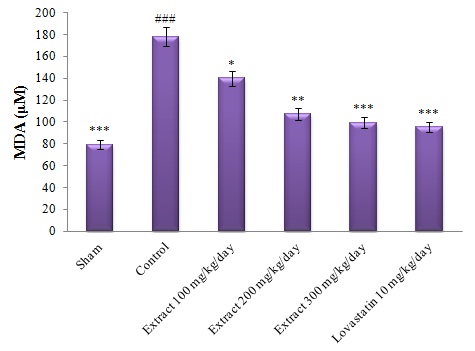
Figure 3- Comparison of SOD levels of red blood cells in different groups.
#: Comparing the control group with the sham group, *: Comparing other groups with the control group. The mean difference is significant at the 0.05 level (#,*: P<0.05).
DISCUSSION
Investigations suggest that saffron petal is among the low-toxin compounds [36], and most studies show that saffron and its extracts have very low or even no toxicity [37-41]. In this study, appropriate and non-toxic doses of this extract were used to treat the animals based on the calculation of the toxic dose of saffron petal extract. Cardiovascular disease remains one of the most important causes of mortality, despite taking many measures to prevent it [42, 43]. Most studies have reported hyperlipidemia as one of the most important causes of atherosclerosis and cardiovascular disease [44, 45]. Thus, the development and discovery of appropriate methods for the control and treatment of hyperlipidemia can be an important step in the reduction and treatment of cardiovascular diseases. In the present study, the administration of hydroalcoholic extract of saffron petal decreased serum levels of TG, cholesterol, and LDL-c and increased serum levels of HDL-c in treated rats compared to the control group.
The hypolipidemic effects of the hydroalcoholic extract of saffron petals in this study were in line with the results of previous studies. For example, Xi et al (2007) showed that crocetin, as one of the compounds in saffron petal extract, reduced blood pressure, epididymal adipose tissue, free fatty acid, TG and LDL, and serum insulin in rats [46]. Sheng et al also concluded that crocin in rats at the dose of 100 mg/kg/day reduced TG, total cholesterol, VLDL, and LDL compared to the control group [47]. The present study also investigated the effect of saffron petal extract on the level of inflammatory markers. In clinical studies, inflammatory markers of CRP, tumor necrosis factor-alpha (TNF-α), β1 interleukin (β1-IL), and IL-6 are changed in the progress of cardiovascular diseases, including coronary artery sclerosis [48, 49]. Increased oxidative stress causes inflammation, which is associated with increased levels of inflammatory markers [50]. Some inflammatory factors, including CRP, increase with increasing cholesterol levels and are an important factor in predicting cardiovascular disease [6]. The present study results also revealed that the hydroalcoholic extract of saffron petal reduced the inflammatory factors and decreased the level of IL-6, CRP, and TNF-α in comparison with the control group. The results of this study are in line with those of previous studies [51-53]. In the study conducted by Xi et al (2005) to induce insulin resistance in rats with dexamethasone or dexamethasone plus crocetin, results showed that serum insulin, free fatty acid, TG, and TNF-α level were significantly lower in the crocetin-receiving group. However, no specific mechanism has been reported for this effect [54].
The saffron extract reduces neuropathic pain caused by severe injury by reducing anti-inflammatory agents such as TNF-α, IL-6, and IL-1β [55]. In a study conducted by Zhang et al, results showed that safranal decreased neural activity and expression of inflammatory cytokines of TNF-α, IL-1β, and increased IL-10 expression after spinal cord injury [56]. Also, in another study, crocin and safranal, which are compounds found in saffron petal extract, inhibited increased levels of inflammatory markers such as TNF-α, and 8-iso-prostaglandin F2a in rats [57]. The destructive effects of oxidative stress are reduced by antioxidant compounds. Many studies have investigated the antioxidant properties of saffron [58-63]. Ardalan et al investigated the antioxidant activity of saffron petals through free radical and 2-diphenyl-1-picrylhydrazyl (DPPH) and showed that saffron petals increased antioxidant content [64]. Studies have indicated that the antioxidant capacity of saffron petals depends on the presence of flavonoids [65]. Flavonoids are compounds that prevent the enzymatic peroxidation of fatty acids and can remove free radicals. Thus, it acts as antioxidant agents. Hence, the most protective effects of saffron petals are attributed to the presence of such compounds that are found abundantly in saffron petals [66]. In the present study, the administration of saffron petal extract reduced SOD and MDA levels and increased antioxidant capacity.
CONCLUSION:
This study revealed that the levels of lipid profile, inflammatory factors, MDA, and SOD increased as a result of consuming high-cholesterol diet and it was found that treatment with saffron petal extract could prevent this increase. It seems that this extract applies this effect by its antioxidant properties so that it increases the level of antioxidants and decreases lipid peroxidation. Also, due to its antioxidant properties, the extract had a significant role in reducing inflammation and, consequently, inflammatory factors. The effects observed in this study may be due to the presence of carotenoids and flavonoids in saffron petals. Thus, further studies are needed to identify these compounds and their mechanism of action.
Competing Interests
The authors declare that they have no competing interests.
ACKNOWLEDGEMENT
The authors of the present study would like to express their gratitude to the research and technology deputyship of the Ilam University of Medical Sciences.
REFERENCES
