
Applied Science Letters


Background: Mosquitoes transmit serious human pathogens, causing millions of deaths every year. The quest for better mosquito control is an endless effort almost throughout the world. Chemical control is a leading method implemented in vector control because of its quick outcome, especially in epidemics. The development of resistance to chemical insecticides resulted in rebounding vectorial capacity. Plants may be alternative sources for mosquito control agents. This study aimed to investigate the toxic effect of the aqueous extract of Acacia nilotica fruits on mosquito larvae under laboratory conditions. Methods: A true experimental study was done to find out the toxic effect of the aqueous extract of Acacia nilotica on mosquito larvae. An aqueous extract from fruits was powdered and strained and was used as a stock solution. Serial concentrations of .0125% up to 2% were tested on mosquito larvae. The larvae were subjected to the extract for 24hrs and the Lc50 was obtained. The trial was repeated three times and the average was obtained. Results: The mortality rate was found to be ranging between 75% and 100%. The average Lc50 for the three trials was found to be 0.004 %. Almost mosquito larvae were Culex species. Conclusion: The aqueous extract of Acacia nilotica could be useful in controlling mosquito larvae, as it is extremely toxic for them. It is recommended that purify the active substance responsible for the toxicity of mosquito larvae and to do more research on the plant extracts in water and other solvents as well as obtaining the plant from different soils.
Toxicity of Water Extract of Acacia Nilotica Fruits against Mosquito Larvae: An Experimental Study
Mohammed Elhag Elkhidr1, Isteshhad Abdo2, Mohamed Madani2, Sara Abdelghani2, Hisham Ali Waggiallah3, Lienda Bashier Eltayeb3*
1 Department of Epidemiology, Faculty of Public and Environmental Health, University of Khartoum, Sudan.
2Department of Parasitology, Faculty of Medical Laboratory Sciences, Al neelain University, Khartoum, Sudan.
3Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Prince Sattam Bin Abdulaziz University, Alkharj, Saudia Arabia.
ABSTRACT
Background: Mosquitoes transmit serious human pathogens, causing millions of deaths every year. The quest for better mosquito control is an endless effort almost throughout the world. Chemical control is a leading method implemented in vector control because of its quick outcome, especially in epidemics. The development of resistance to chemical insecticides resulted in rebounding vectorial capacity. Plants may be alternative sources for mosquito control agents. This study aimed to investigate the toxic effect of the aqueous extract of Acacia nilotica fruits on mosquito larvae under laboratory conditions. Methods: A true experimental study was done to find out the toxic effect of the aqueous extract of Acacia nilotica on mosquito larvae. An aqueous extract from fruits was powdered and strained and was used as a stock solution. Serial concentrations of .0125% up to 2% were tested on mosquito larvae. The larvae were subjected to the extract for 24hrs and the Lc50 was obtained. The trial was repeated three times and the average was obtained. Results: The mortality rate was found to be ranging between 75% and 100%. The average Lc50 for the three trials was found to be 0.004 %. Almost mosquito larvae were Culex species. Conclusion: The aqueous extract of Acacia nilotica could be useful in controlling mosquito larvae, as it is extremely toxic for them. It is recommended that purify the active substance responsible for the toxicity of mosquito larvae and to do more research on the plant extracts in water and other solvents as well as obtaining the plant from different soils.
Keywords: Water Extract, Acacia Nilotica, Mosquito Larvae.
INTRODUCTION
More than two billion people, mostly in tropical countries are at risk of mosquito-borne diseases such as malaria, dengue, hemorrhagic fever, and filariasis [1, 2]. These infectious diseases mainly affect the tropic’s poorest people. An estimated 50 million people are infected with dengue each year [3]. Malaria has a crippling effect on Africa’s economic growth and perpetuates vicious cycles of poverty [4]. Approximately 300–500 million clinical cases and >1 million deaths are recorded every year [5]. The responsible pathogens are transmitted through bites of blood-sucking mosquitoes on the populations in tropical and subtropical regions [6]. The genera Culex, Aedes, and Anopheles are the most important vectors involved in disease transmission to humans. Although there are proven strategies to control mosquito-borne diseases, mosquitoes still cause a huge public health problem in Africa. Across African people are exposed to mosquito bites because the larval habitats are widely distributed in humid areas such as flood areas and rice farms [7-10]. These sites with larvae might be altered to decrease the mosquito population for the interruption of disease transmission [11-13]. Larviciding is a successful way of reducing mosquito densities in their breeding places before they emerge into adults. Pesticides are indeed very effective in its use. However, the use of chemical insecticides is often toxic to both human and non-target animals. The intensive use of chemical insecticides led to the development of resistant insect populations resulting in reduced control, environmental pollution hence leading to bio-amplification in the food chain and contamination.
MATERIALS AND METHODS:
Study design:
An experimental study was carried to find out the toxic effect of the aqueous extract of Acacia nilotica on mosquito larvae from October 2017 to October 2019. The study conducted at the faculty of medical laboratory sciences at Al neelain university and ethical approval was taken from the institutional review board from faculty. For quality control, all collected Mosquitos larvae were checked for their species as well as viability by an experienced Entomologist.
Methodology:
Larvae collection and examination: larvae were collected from Khartoum Bahry/Sudan (Alfaky Hashem village) and transported to the laboratory with utmost care avoiding drowning of larvae. By using a dissecting microscope all larvae were examined for species and their viability before starting laboratory work.
Acacia nilotica fruits preparation:
Acacia nilotica fruits were purchased from the local market, then dried in a shaded place, and were powdered using a clean electric blender. 2% (W/V) stock solution of Acacia nilotica was prepared by adding 20 grams of the fruit powder to 800 ml of distilled water for 24hours at room temperature (37oC). The extract was then strained and the volume was adjusted to 1000ml with distilled water to give a stock solution extract of 20grams/liter i.e. 20,000 ppm of the powdered fruit material. Further serial dilutions were done from the above stock solution to which snails were exposed i.e. 20,000/10,000/ 5,000/ 1,000/500/ 250 and 125ppm.
Twenty larvae were placed in small cups containing 50ml of each concentration (20,000, 10,000, 5,000, 1,000, 500, 250 and 125ppm). Also, twenty larvae were put in a separate cup containing 50ml of distilled water as a control. Reading of mortality was done after 24hours counting the dead and live ones. The larvae were considered dead when settling on the bottom without moving. The experiments were conducted at room temperature (30-37oC), using a mercuric thermometer. Each experiment was repeated three times. Plastic dishes of 1000 ml capacity, strainers, cups, Flasks, Measuring cylinders, wooden sticks, electric blender, normal graph papers, and a scientific calculator were all material used in the experiment.
Analysis:
Mortality was then transferred into probit transformation units and tabulated against the logarithm of concentrations of the water extract.
RESULTS:
The results of the experiments were analyzed using the analysis of variance and log probit transformation. A normal graph was used instead of the logarithmic graph because of the unavailability of the logarithmic scale graph papers. Data for each replicate was obtained. The average mortality of mosquito larvae subjected to aqueous extract of Acacia nolitica for the three trials ranged between 90%-75% (Probit units were between 6.28 -5.67) and the concentration ranged between 5000 to 125 ppm. The average Lc50 for the three trials was found to be 0.0051, 0.004, and 0.004 respectively. The average Lc50 was found to be 0.0044.
Table 1: Mortality of mosquito larvae subjected to water extract of Acacia nilotica fruits (trail 1), Khartoum, 2017.
|
PPM |
Conc. % |
Live |
Dead |
Log Concentration |
% Mortality |
Probit unit |
Prop(p) |
Correct (p) |
Log it (p) |
|
20,000 |
2 |
0 |
20 |
0.30 |
100% |
|
1 |
1 |
|
|
10,000 |
1 |
0 |
20 |
0.00 |
100% |
|
1 |
1 |
|
|
5000 |
.5 |
0 |
20 |
-0.30 |
100% |
|
1 |
1 |
|
|
1000 |
.1 |
1 |
19 |
-1.00 |
95% |
6.64 |
0.95 |
0.95 |
2.94 |
|
500 |
.05 |
3 |
17 |
-1.30 |
85% |
6.04 |
0.85 |
0.85 |
1.73 |
|
250 |
.025 |
5 |
15 |
-1.60 |
75% |
5.67 |
0.75 |
0.75 |
1.10 |
|
125 |
.0125 |
5 |
15 |
-1.90 |
75% |
5.67 |
0.75 |
0.75 |
1.10 |
|
0 |
0 |
20 |
0 |
|
0% |
|
0 |
|
|
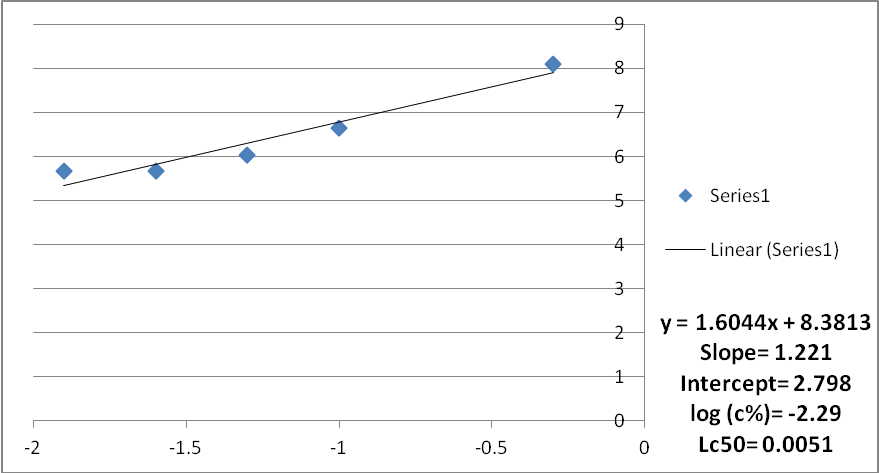
Fig. 1: Mortality curve of mosquito larvae subjected to water extract of Acacia nilotica fruits (Trial 1)
Table (2): Mortality of mosquito larvae subjected with water extract of Acacia nilotica fruits (trail 2), Khartoum, 2017
|
Conc. PPM |
Conc. % |
Live |
Dead |
Log Concentration on |
% Mortality |
Probit unit |
Prop(p) |
Correct (p) |
Log it (p) |
|
10000 |
1 |
0 |
20 |
0.00 |
100% |
|
1 |
1 |
|
|
5000 |
0.5 |
0 |
20 |
-0.30 |
100% |
|
1 |
1 |
|
|
1000 |
0 .1 |
3 |
17 |
-1.00 |
85% |
6.04 |
0.85 |
0.85 |
1.73 |
|
500 |
0 .05 |
3 |
17 |
-1.30 |
85% |
6.04 |
0.85 |
0.85 |
1.73 |
|
250 |
0 .025 |
5 |
15 |
-1.60 |
75% |
5.67 |
0.75 |
0.75 |
1.10 |
|
125 |
0.0125 |
5 |
15 |
-1.90 |
75% |
5.67 |
0.75 |
0.75 |
1.10 |
|
0 |
|
20 |
0 |
|
|
|
0 |
0 |
|
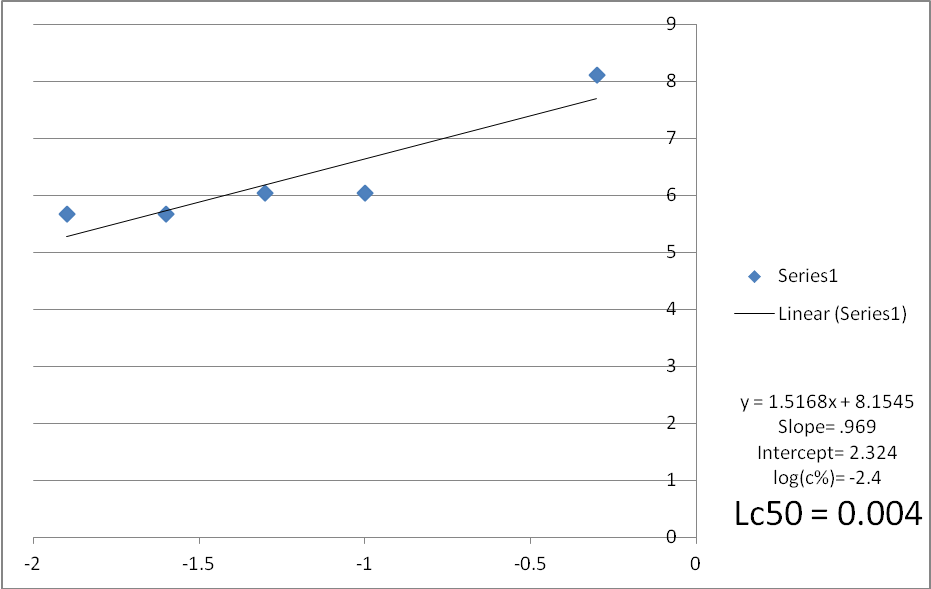
Fig. 2: Mortality curve of mosquito larvae subjected to water extract of Acacia nilotica fruits (Trial 2)
Table 3: Mortality of mosquito larvae subjected to water extract of Acacia nilotica fruits (trail 3), Khartoum, 2017
|
Conc. PPM |
Conc. % |
Live |
Dead |
Log Concentration on |
% Mortality |
Probit unit |
Prop (p) |
Correct (p) |
Log it (p) |
|
5000 |
0 .5 |
0 |
20 |
-0.30 |
100% |
|
1 |
1 |
|
|
1000 |
0.1 |
2 |
18 |
-1.00 |
90% |
6.28 |
0.90 |
0.90 |
2.20 |
|
500 |
0.05 |
3 |
17 |
-1.30 |
85% |
6.04 |
0.85 |
0.85 |
1.73 |
|
250 |
0.025 |
3 |
17 |
-1.60 |
85% |
6.04 |
0.85 |
0.85 |
1.73 |
|
125 |
0.0125 |
6 |
14 |
-1.90 |
70% |
5.52 |
0.70 |
0.70 |
0.85 |
|
0 |
|
20 |
0 |
|
0% |
|
0 |
|
|
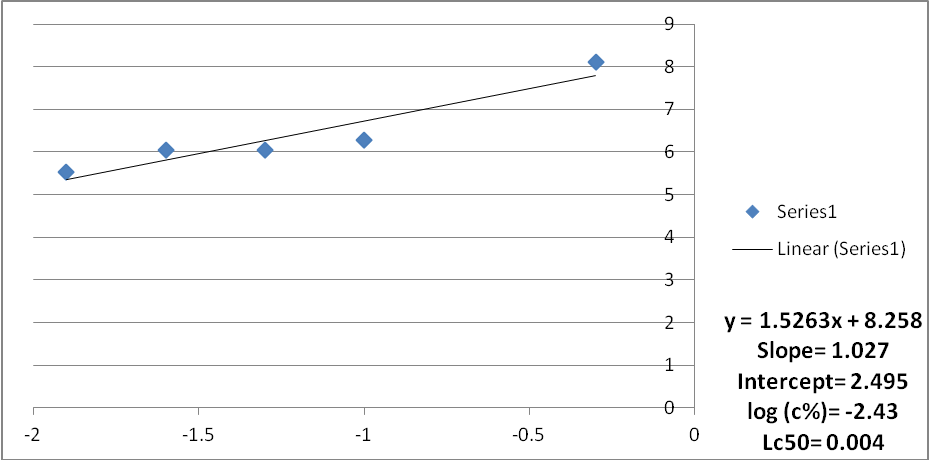
Fig. 3: Mortality curve of mosquito larvae subjected to water extract of Acacia nilotica fruits (Trial 3)
Table (4): Mortality of mosquito larvae subjected with water extract of Acacia nilotica fruits (Average of the three trials), Khartoum, 2017
|
PPM |
Conc.% |
Log Concentration |
Prop, P |
Corr, P |
logit (p) |
Probit unit |
Mortality % |
|
|
20.000 |
2 |
0.30 |
1 |
1 |
100% |
|||
|
10.000 |
1 |
0.00 |
1 |
1 |
100% |
|||
|
5000 |
.5 |
-0.30 |
1 |
1 |
100% |
|||
|
1000 |
.1 |
-1.00 |
0.90 |
0.90 |
2.20 |
6.28 |
90% |
|
|
500 |
0.05 |
-1.30 |
0.85 |
0.85 |
1.73 |
6.04 |
85% |
|
|
250 |
0.025 |
-1.60 |
0.80 |
0.80 |
1.39 |
5.84 |
80% |
|
|
125 |
0.0125 |
-1.90 |
0.75 |
0.75 |
1.30 |
5.67 |
75% |
|
|
0 |
0 |
0 |
0% |
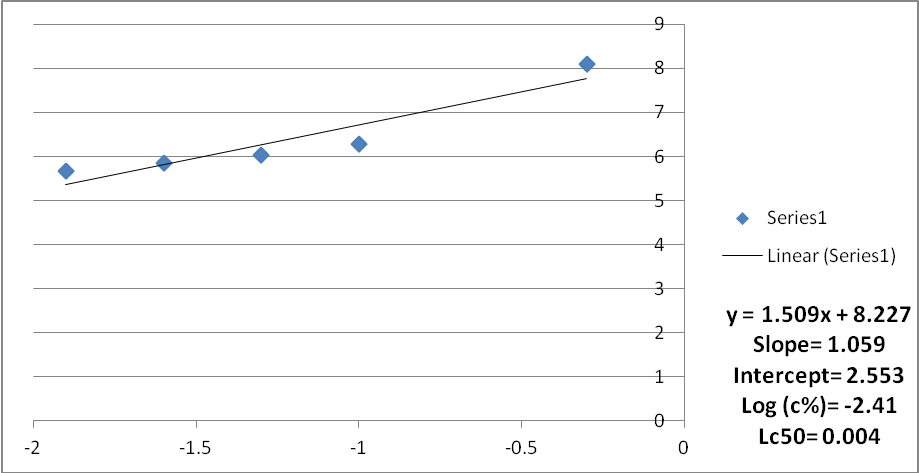
Fig. 4: Mortality curve of mosquito larvae subjected to water extract of Acacia nilotica (Average mortality of the three trials)
DISCUSSION:
The need for using natural products in the control of mosquito larva necessitated continuous research in this field especially products of plant origin. This effort aimed at finding the effect of the aqueous extract of Acacia nilotica on mosquito larvae. Many studies in Sudan were conducted with other local plants but there is no published research on the use of Acacia nilotica. This shrub is quite popular in most parts of Sudan. It is an evergreen shrub and may be grown in different soils and different climates. It is also important to note that the nature of soil may give different concentrations of the active ingredient. Furthermore, the extracts of Acacia nilotica seeds or bark may give different values. The LC 50 values seem to be reasonable when compared with some standard insecticides already used as larvicides.
So few references have been developed to Acacia nilotica entomological behavior and there are no indications of mosquitoes. Our finding comes in contact with Chaubal R et al [14] which the raw extracts of A nilotica were therefore checked for their biological activity toward mosquito larvae, the noted that an acetone extract at 500-ppm concentration demonstrated chronic toxicity to Aedes aegypti and Culex quinquefasciatus IV instar mosquito larvae
Extraction and standardization of the active ingredient are important. The use of extracts from different types of soils on different strains of larvae as well as those from other parts of the plant are important to come out with a solid LC50. Studies on the toxic effect of extracts that are soluble in solvents other than water are also recommended [15], since it is extracted from the herb, is environmentally, economically appropriate, non-hazardous to non-target species, and would be harmless, unlike other commercially available insecticides.
CONCLUSION:
The aqueous extract of Acacia nilotica could be useful in controlling mosquito larvae, as it is extremely toxic for them. It is recommended that purify the active substance responsible for the toxicity of mosquito larvae and to do more research on the plant extracts in water and other solvents as well as obtaining the plant from different soils. Further studies on the toxic effect of extracts that are soluble in solvents other than water are contraindicated since it is extracted from the herb, is environmentally, economically appropriate, non-hazardous to non-target species, and would be harmless, unlike other commercially available insecticides.
ACKNOWLEDGEMENT:
This publication was supported by the Deanship of scientific research at Prince Sattam bin Abdulaziz University. The authors appreciated to Faculty of Medical Laboratory Sciences at Al Neelain University.
Conflict of interest statement:
The authors declare that they have no conflict of interest.
Authors contribution:
REFERENCES
